Required equipment for registration of advanced regenerative medical implementation institution
It provides cell separation, incubator, storage facility, shaking culture machine, centrifuge, etc.
We consult on processes for movement, storage, and processing.

Providing documents and samples for registration of advanced regenerative medical practice institutions
I have a lot of experience designating it as an implementation agency, so I fill out and provide documents that are difficult to fill out at the hospital.
| registration documents | content |
|---|---|
| Standard Work Instructions (SOP) | Provides permission to obtain permission |
| Cell Supply Agreement for Cell Processing Facilities | Provide contract preparation |
| 1. Application for Designation of Advanced Regenerative Medicine Implementation Agency | Attachment to the Rules on Safety and Support for Advanced Regenerative Medicine[Form 1] |
| 2. Institutional Status Summary Table | |
| 3. Copy of a medical institution's license | |
| 4. Submission of facility, equipment, and manpower status | Regulations on the designation of advanced regenerative medical practice institutions and compliance with cell processing work |
| 5. Facilities and equipment pre-check checklist | 32 items |
| 6. Supplemental data related to facility and equipment checklist | Test report, etc |
| 7. Workforce Precheck Checklist | 12 items |
| 8. Supplemental data related to manpower checklist | 32 items |
| 9. Standard Work Instructions Precheck Checklist |
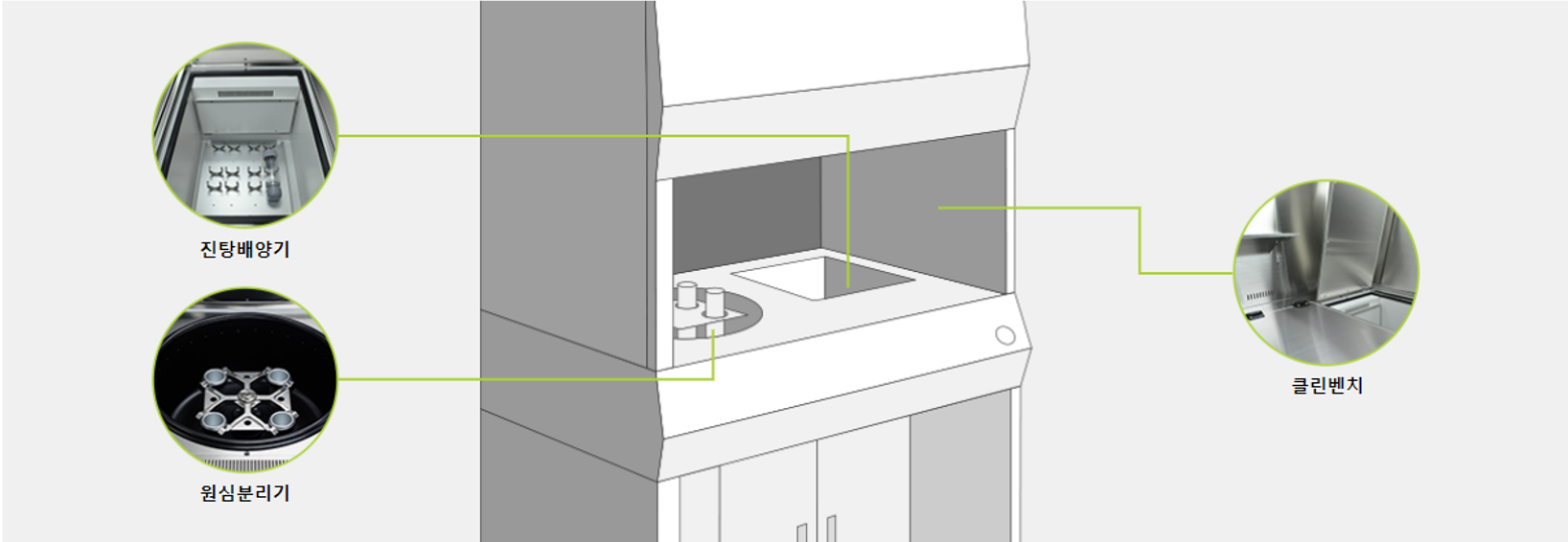
Multi-workstation for SVF isolation
Centrifuges and shaking cultures can be used within the sterile workbench to speed up the flow and the entire process of SVF separation takes place in a sterile environment, reducing the risk of external contamination for more convenient and safe stem cell extraction.

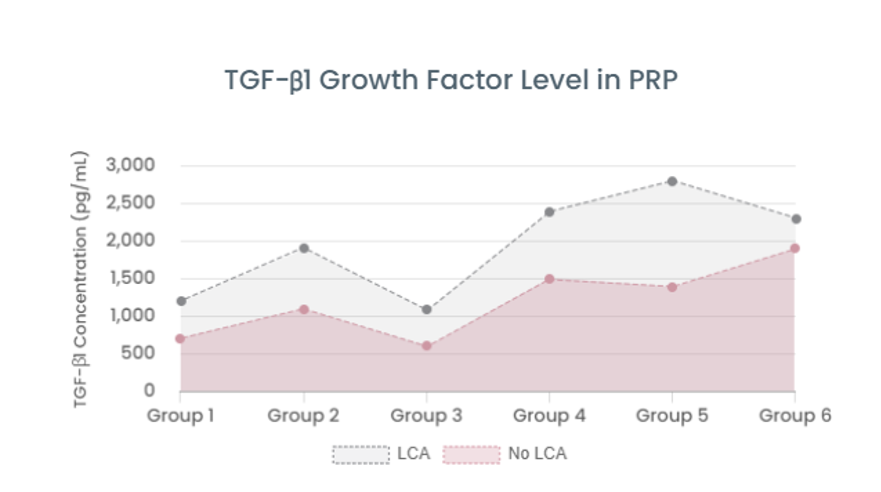
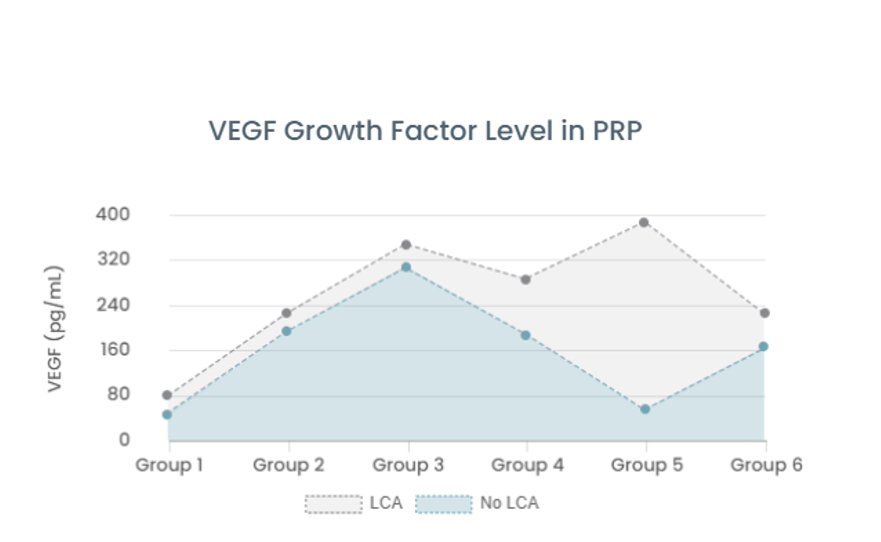
Consulting Performance of Advanced Regenerative Medicine Implementing Institutions
Many university hospitals, general hospitals, and clinics have received permission from the Ministry of Health and Welfare.
Since the equipment we manufacture is officially registered with the Ministry of Food and Drug Safety, we can quickly obtain permission without any special examination of the equipment.
Q&A
Q1: How much space is needed to be designated as an advanced regenerative medical implementation institution?
If you have an operating room, you can use the existing spaces without the need for additional space.
Q2: How long will it take to be designated as an implementation agency by the Ministry of Health and Welfare?
It takes about six to nine months.
Q3: How much does it cost to set up advanced regenerative care?
It is 180 million won (VAT-specific) including equipment, facilities, and all document agency consulting fees
Q4: Do you need additional manpower?
You don't need new personnel because you can also hold a concurrent position.
Q5: What facilities and equipment do you need?
Adipocyte cell separation equipment, incubator, storage facilities (refrigerated, frozen), cell actuator, centrifuges, etc (Essential equipment to be designated is included in the consulting fee) .



