Mirae Biogroup is
a biotechnology enterprise at the forefront of the world’s first stem cell and immune cell technologies, specializing in advanced cellular therapeutics research.
Mirae Biogroup is an institution officially licensed and recognized for its safety and highest quality cellular technology by Korea's Ministry of Food and Drug Safety and Japan's Ministry of Health, Labour and Welfare. It conducts comprehensive research in adult stem cells, immune cells, and anti-aging, and develops various cellular therapies.
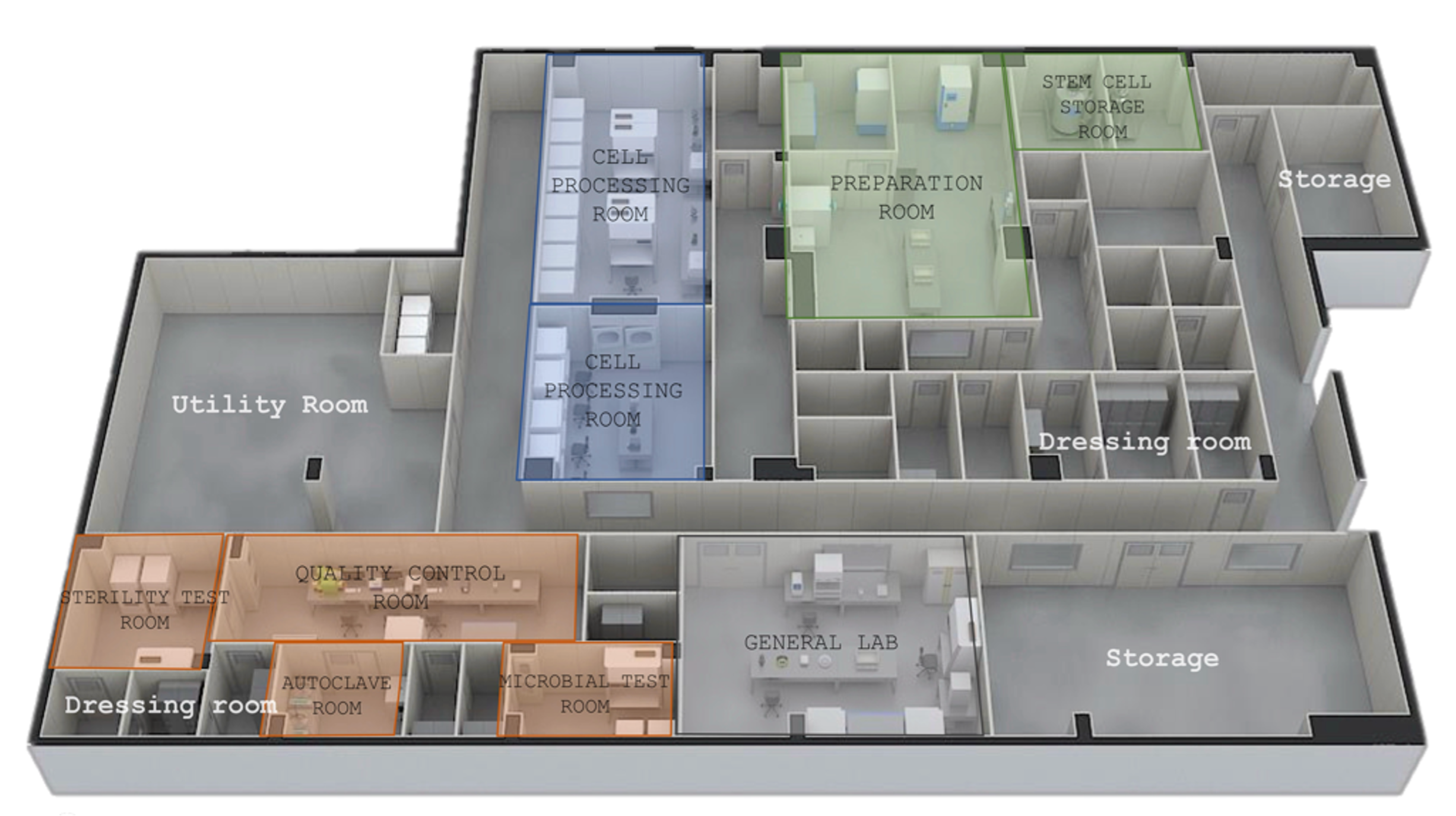


The Cell Processing Facility (CPF) is a specialized facility that isolates, cultures, and stores cells such as stem cells and immune cells. It primarily cultures, manipulates, and processes living cells for medical use, aimed at patient treatment.
Mirae Biogroup's Cheongdam Center operates directly with medical and research staff, as well as clinical research professionals, adhering to international standards to ensure strict quality control supervision.



Mirae Biogroup GMP Research Laboratory




Key Facility Features
The Rating of Mirae Biogroup’s Cell Processing Facility (Cleanliness Rating, Cleanroom Rating)
The Mirae Biogroup's Cell Processing Facility (CPF) is crucial for the manufacture and processing of cellular therapeutics, immune cell therapies, and gene therapies, necessitating the maintenance of a sterile environment.
The facility’s cleanliness is regulated according to GMP standards, operating cleanrooms ranging from Class 100 to Class 100,000.
| USA Standard | European Standard | No. of Particles (≥0.5 µm, pcs/m³) | Application Example |
|---|---|---|---|
| Class 100 | Grade A | ≤ 3,520 | laminar airflow workbenches and cell processing |
| Class 1,000 | Grade B | ≤ 35,200 | sterile manufacturing labs |
| Class 10,000 | Grade C | ≤ 352,000 | culturing, manipulation, and the manufacture of cell therapy products |
| Class 100,000 | Grade D | ≤ 3,520,000 | general manufacturing zones and entryways |
Grade A Areas where asepsis is essential (e.g., injection filling, cell infusion stages)
Grade B Areas surrounding Grade A zones
Grade C, D Cell handling and general manufacturing areas

















